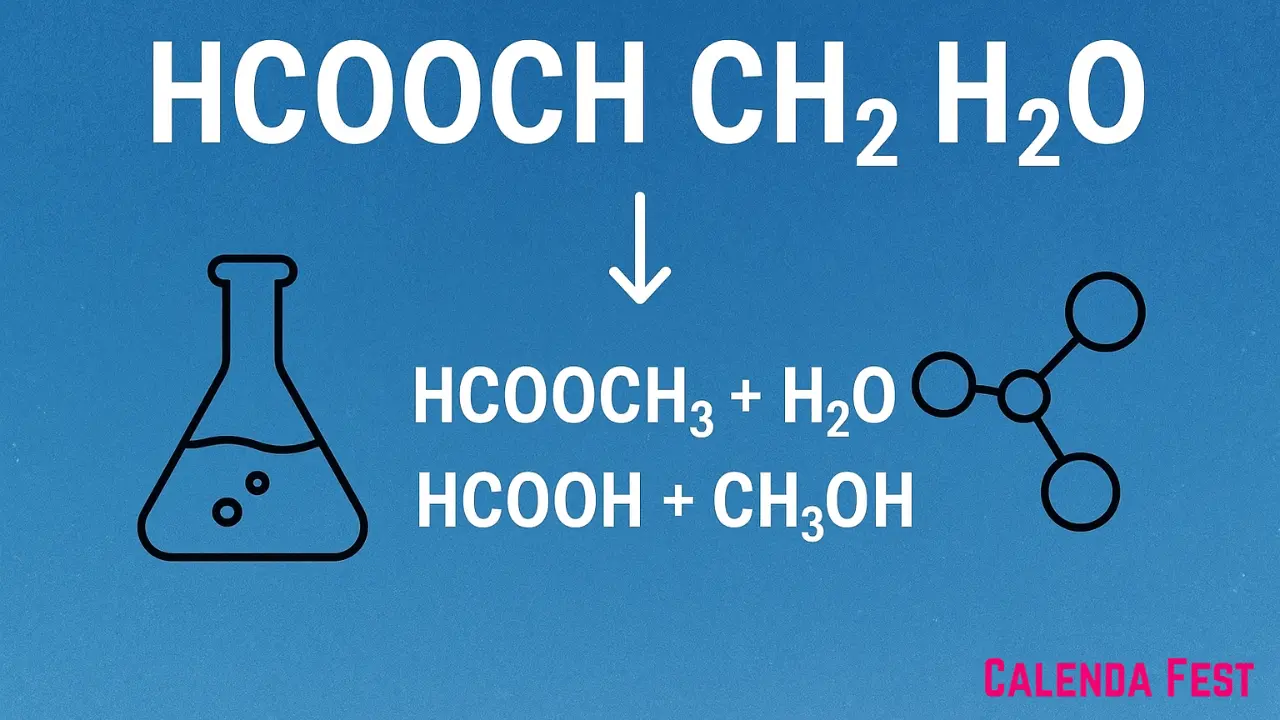
Chemistry often presents formulas and reactions that may appear complex at first glance, yet they carry immense value in both theoretical studies and practical applications. One such expression is hcooch ch2 h2o, which has sparked curiosity due to its unusual notation and its connection to the well-known ester hydrolysis reaction. At its core, this combination refers to the interaction of methyl formate (commonly written as HCOOCH₃) with water (H₂O), which undergoes hydrolysis to produce formic acid (HCOOH) and methanol (CH₃OH). While the formula may sometimes be written in non-standard form, the underlying chemistry demonstrates a fundamental principle in organic reactions: the breaking of ester bonds with the addition of water. To understand this process more deeply, it is necessary to explore not only the meaning of the components within hcooch ch2 h2o but also the broader context of ester hydrolysis, the role of intermediates such as methylene groups, and the significance of the resulting compounds in everyday industries. By unpacking these aspects, we gain both clarity about the formula itself and appreciation for its real-world relevance.
The phrase hcooch ch2 h2o brings together three key parts that can be explained in detail. The first is HCOOCH, which is shorthand for methyl formate, an ester that arises from formic acid and methanol. This liquid is colorless, highly volatile, and has an odor often described as fruity or pungent. It is widely used as a solvent, in chemical synthesis, and even in specialized fumigation processes. The second part, CH₂, represents the methylene group, a highly reactive fragment of organic molecules that often appears in intermediate steps of reactions. While free methylene (CH₂) rarely exists in isolation, its inclusion in chemical shorthand reflects the structural components or reactive sites involved in hydrolysis or rearrangement. Finally, the inclusion of H₂O emphasizes the central role of water in breaking ester bonds. Water is not just a solvent in this reaction; it actively participates by splitting the ester into its acid and alcohol components. This combination gives the phrase its unique identity, summarizing in a compact way the interaction of an ester with water and highlighting the chemical transformation that results.
The Chemistry Behind Ester Hydrolysis and the Reaction Pathway
When analyzing the details of hcooch ch2 h2o, the primary focus falls on ester hydrolysis, which is among the most important reactions in organic chemistry. Esters are compounds formed from the reaction of an alcohol with an acid, and they contain a characteristic –COO– linkage. In the case of methyl formate, this ester consists of the formyl group (derived from formic acid) bonded to a methoxy group (derived from methanol). Hydrolysis involves breaking this bond by introducing water, often under acidic or basic conditions. Under acid catalysis, water donates a proton to make the carbonyl group more susceptible to nucleophilic attack, after which the molecule rearranges and splits into formic acid and methanol. Under basic conditions, hydroxide ions attack the carbonyl carbon directly, leading to saponification, a process that irreversibly produces the salt of formic acid and methanol. This dual pathway demonstrates the versatility of hydrolysis, making it a cornerstone reaction in both laboratory practice and industrial applications.
The products of this hydrolysis are as significant as the process itself. Formic acid (HCOOH) is a simple but highly useful organic acid. It is found in nature in the venom of ants and is employed industrially in leather processing, textile dyeing, and as a preservative due to its antibacterial properties. In recent years, it has gained attention in the field of renewable energy, particularly as a hydrogen storage material in fuel cells. Methanol (CH₃OH), the other product, is an essential solvent and feedstock in countless chemical syntheses, as well as an alternative fuel. Both products illustrate why the reaction symbolized by hcooch ch2 h2o matters: it connects a relatively simple ester to compounds that are indispensable in modern industries. By studying such transformations, chemists deepen their understanding of molecular behavior while industries gain pathways to economically valuable materials.
Properties, Uses, and Industrial Importance of Methyl Formate and Its Derivatives
Methyl formate, the key ester behind hcooch ch2 h2o, is far more than a chemical curiosity. Its physical properties make it extremely versatile. It is a clear liquid with a boiling point of about 31.5 °C, which means it evaporates quickly at room temperature. This volatility, combined with its solvent characteristics, allows it to dissolve a range of resins, oils, and other organic substances. In industry, methyl formate is used to produce formamide and dimethylformamide, which are critical intermediates in plastics, pharmaceuticals, and agrochemicals. Its ability to act as a building block for larger molecules ensures it remains a staple in chemical manufacturing. Additionally, because methyl formate breaks down relatively quickly in the atmosphere, it is often regarded as less environmentally persistent than other industrial solvents, though its flammability and potential toxicity require strict handling procedures.
The importance of the hydrolysis products cannot be overstated. Formic acid, as mentioned, plays a key role in traditional industries such as leather tanning and fabric processing, where it assists in dye fixation and pH control. Its antibacterial nature also makes it useful in animal feed preservation. In advanced fields, formic acid is gaining momentum as a green energy carrier, as it can decompose into hydrogen and carbon dioxide under controlled conditions, offering a promising route for hydrogen fuel technologies. Methanol, on the other hand, has become central in energy discussions as well. It is used in biodiesel production, as a fuel in specialized engines, and as a precursor to formaldehyde, acetic acid, and numerous plastics. The fact that one reaction, summarized in the shorthand hcooch ch2 h2o, leads to the formation of both methanol and formic acid illustrates the remarkable industrial significance of even the simplest organic processes. In this way, the reaction is not just an academic concept but a gateway to products that shape daily life.
The Role of Methylene Groups and the Importance of Accurate Nomenclature
One striking part of the expression hcooch ch2 h2o is the presence of CH₂, which requires some clarification. In organic chemistry, CH₂ represents the methylene group, a fragment with two hydrogens bonded to a carbon atom that still has two available bonding sites. It often acts as a bridge in molecular chains or appears in reaction intermediates. While free methylene is unstable under normal conditions, stabilized forms and methylene-containing compounds are central to synthetic organic chemistry. Methylene groups can influence reactivity, provide flexibility in molecular structures, and play roles in rearrangements or chain extensions. Thus, even if the inclusion of CH₂ in the shorthand may be non-standard, it highlights the importance of structural fragments in understanding reactions at a deeper level. For students and professionals alike, recognizing these groups sharpens awareness of how molecules behave and transform.
Equally important is the broader lesson of nomenclature. Chemistry relies on precise language to prevent confusion and ensure accurate communication. Writing formulas such as hcooch ch2 h2o can sometimes obscure the true identities of the compounds involved. The standard way of expressing the reaction would be HCOOCH₃ + H₂O → HCOOH + CH₃OH, which leaves no ambiguity about the reactants or products. However, because shorthand or informal notations often appear in notes, discussions, or simplified diagrams, misunderstandings can occur. This makes it vital for students and practitioners to double-check formulas and ensure correct representation. Beyond academics, incorrect formulas can create risks in industrial settings, where miscommunication could lead to unsafe handling or improper reactions. Therefore, one lasting takeaway from examining this expression is the necessity of precision: while shorthand is convenient, accuracy protects both understanding and safety in the field of chemistry.
Conclusion
The expression hcooch ch2 h2o may at first seem puzzling, but closer examination reveals a wealth of chemistry and practical importance hidden within it. It represents the hydrolysis of methyl formate in the presence of water, leading to the production of formic acid and methanol. This reaction, fundamental in organic chemistry, demonstrates how esters break apart and how water actively participates in creating new compounds. The process not only deepens theoretical knowledge but also has far-reaching industrial applications, as both formic acid and methanol are essential in modern manufacturing, energy research, and daily life. Along the way, the inclusion of CH₂ highlights the importance of recognizing functional groups and intermediates, while the formula itself underscores the value of precise chemical nomenclature. Ultimately, studying this reaction provides both insight into molecular transformations and appreciation for the role of simple chemical processes in shaping industries and innovations.
FAQs
1. What does the formula hcooch ch2 h2o represent?
It represents the hydrolysis of methyl formate (HCOOCH₃) with water, producing formic acid (HCOOH) and methanol (CH₃OH).
2. Why is ester hydrolysis important in chemistry?
Ester hydrolysis is a fundamental reaction that demonstrates how water breaks ester bonds, leading to valuable products like alcohols and acids used in industries and research.
3. What are the main uses of methyl formate?
Methyl formate is used as a solvent, in chemical synthesis of intermediates like formamide, and in some fumigation processes due to its volatility.
4. How are formic acid and methanol useful in daily life?
Formic acid is used in leather processing, textiles, and energy research, while methanol serves as a solvent, industrial feedstock, and alternative fuel source.
5. Why is chemical nomenclature important when discussing reactions like hcooch ch2 h2o?
Precise chemical naming avoids confusion, ensures safety in industrial contexts, and allows clear communication between scientists and students about the actual compounds involved.